Abstract
Up to 90% of MDS patients require red blood cell (RBC) transfusions. Literature addressing incidence and impact of alloimmunization in MDS is limited. We previously reported that 11% RBC-transfused MDS patients develop alloantibodies and RBC-transfusion requirement increases following alloimmunization (Singhal et al Haematologica 2017).
This study aims to assess mechanism of increased RBC transfusion requirement following alloimmunization in MDS patients by comparing RBC-transfusion requirement following single and multiple alloantibodies, and impact of autoantibody on transfusion requirement.
Primary MDS (PMDS), oligoblastic acute myeloid leukemia (AML) and therapy-related myeloid neoplasm (t-MN) patients enrolled in the SA-MDS registry (n=1002) between Nov 1991-Jun 2017, followed up for >3 months, received at least 1 unit of RBC and did not develop alloantibodies before first RBC transfusion were selected for analysis. Cumulative incidence (CI) of RBC-alloimmunization and clinical impact of alloimmunization including autoantibody formation and change in RBC-transfusion requirements was assessed. We also assessed risk factors for alloimmunization using recursive partitioning and Cox-regression.
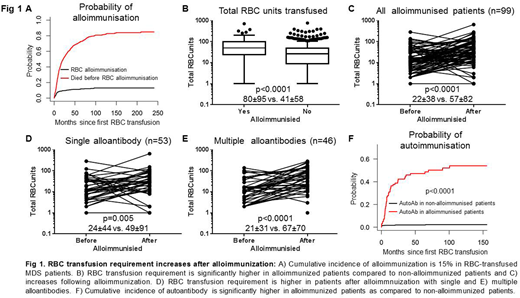
Seven hundred and sixty-two patients (76%) were eligible for analysis; 584 (76.5%) PMDS, 56 (7.3%) oligoblastic AML and 123 (16%) were t-MN. The median age was 72 years (range 18-97) and 489 (64%) were males. According to the Revised International Prognostic Scoring System (IPSS-R), 44.9% and 54.9% patients were classified as IPSS-R Very low/Low risk and Intermediate/High/Very high risk, respectively. The CI of alloimmunization in RBC-transfused patients was 15% (Fig 1A) and alloantibodies were most commonly against K (32%), E (26%), C (18%), Jka (10%) & Duffy (3%) antigens. Interestingly, 53% (53/99) of alloimmunized patients had single alloantibody while 46% (46/99) had multiple alloantibodies detected simultaneously or subsequently.
RBC requirement was significantly higher in alloimmunized compared to non-alloimmunized patients (80±95 vs 41±58, p<0.0001; Fig 1B). This difference is unlikely due to difference in overall survival (OS) as median OS of the two groups was not significantly different (27.5 vs 33.7 months; p=0.2). Importantly, RBC requirement of alloimmunized patients significantly increased after alloantibody formation (22±38 vs 57±82, p<0.0001; Fig 1C). This increase is unlikely to be due to difference in follow-up period before and after alloimmunization (12.3 vs 10.7 months; p=0.9).
RBC transfusion requirement increased following single (24±44 vs 49±91; p=0.005; Fig 1D) and multiple (21±31 vs 67±70; p<0.0001; Fig 1E) alloantibody formation. RBC requirement prior to alloimmunization was not significantly different between patients with single and multiple alloantibodies (24±44 vs 21±31; p=0.9); however following alloimmunization, it was higher in patients with multiple alloantibodies compared to single alloantibody (49±91 vs 67±70; p=0.06).
Increase in RBC requirement following alloimmunization could partly be due to autoantibody formation.The 12-month CI of autoantibody formation was significantly higher in alloimmunized patients compared to non-alloimmunized (31 vs 1.7%; p<0.0001; Fig 1F) and 26% of autoantibodies were detected within 3 months of alloimmunization indicating temporal relation between alloimmunization and autoantibody formation. Within alloimmunized patients, more patients with multiple alloantibodies developed autoantibodies as compared to patients with single alloantibody (67% vs 31%; p<0.001). We then compared impact of autoantibodies on RBC transfusion requirements. Baseline RBC requirement in alloimmunized patients with and without autoantibodies was not significantly different (25.4±34.6 vs 20.4±41.5; p=0.2). However, RBC transfusion requirement following alloimmunization was significantly higher in patients developing autoantibodies compared to patients without autoantibodies (82.51±110 vs 35.61±35.93; p=0.003).
In our large cohort of 1002 patients, 15% of RBC transfused patients develop alloantibodies, most commonly against Rh and Kell. The RBC transfusion requirement increases after alloimmunization, most probably due to formation of multiple subsequent alloantibodies and autoantibodies. Hence, we recommend extended phenotype matched RBC transfusion to MDS patients.
Ross:Celgene: Research Funding; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS: Honoraria. Hiwase:Celgene: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal